

· ISO 13485 Certificate
· A full set of interventional catheter and low-temperature plasma electrode tip manufacturing equipment

· Factory area of 2,000 m2 (two sites)
· Class 10,000 clean workshop of 500 m2

· Sound physical, chemical and microbiological laboratories
· Independent product test system
· Independent material unpacking, incoming inspection, packaging, quality inspection, warehouse and other areas
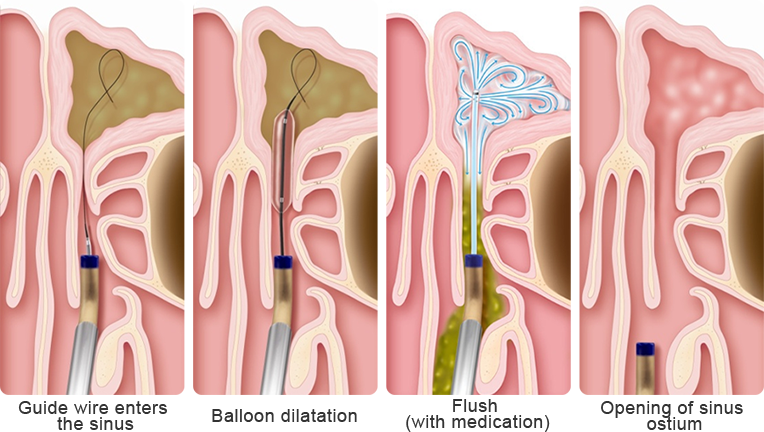
Features
no cartilage tissue is removed
virtually no risks or complications
rapid relief of symptoms
within 24 hours

• Sinus balloon catheter
• Sinus guiding catheter
• Sinus guide wire (luminous)
• Sinus flushing catheter
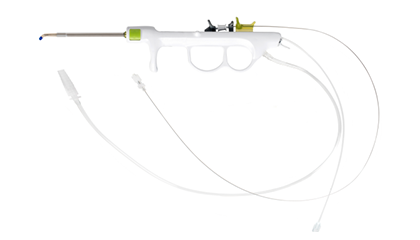
• Integration of of guiding, flushing and balloon dilation function
• Quick and easy to use, shortening the operation time
• Greatly facilitates the operator and reduces the patient's discomfort
•
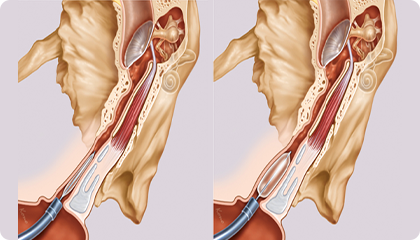
• The balloon has 16 sizes, up to 4.0 mm
• Better adaptability to different clinical needs
• The balloon is non-compliant, with good pressure-bearing capability
• The balloon crimp diameter is only 0.7 mm to facilitate passage through narrower diseased area
•

• 12 sizes to keep airway unobstructed
• Rapid relief of OSAS (sleep apnea syndrome)
• Easy to use with little foreign body sensation
•

• Plasma knife ENT series
• Plasma knife orthopedic series
• Plasma knife urology series
• Plasma knife ophthalmic series
• 9 series, a total of 36 specifications/models